Theme XVIII: Heterocycles
Porphyrin. Porphyrin ring.
Porphyrin. What are porphyrins or
porphyrin ring? Characteristics, properties and importance of Porphyrin.
As stated previously, the porphyrin ring is just a tetrapyrrole nucleus from the structure of chlorophyll.
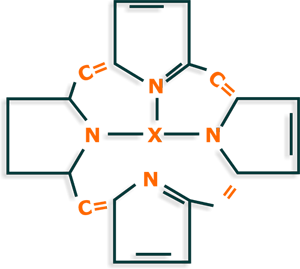
The compounds formed by the four pyrrole rings linked in the α position by methine bridges are called porphin; derivatives thereof are called porphyrins or porphyrin
ring.
Porphines not occur naturally but porphyrins are widely distributed in nature, for example complexed with iron in hemoglobin and cytochromes.
Porphyrin
As shown in the porphyrin ring are four pyrrole rings linked by methine groups and 9 of these bonds are conjugated links (one single bond and one double).
This high electronic conjugation gives great stability to the porphyrin ring and in turn, makes these compounds possess an intense color increased by the central atom, either
iron (hemoglobin) or magnesium (in chlorophyll).
see also
Pentagonal heterocyclic compounds with one heteroatom
Pyrrole
Chlorophyll
Hexagonal heterocyclic compounds: The Pyran and Pyrimidine
Pyrimidine ring
Indole, tryptophan, indole-3-acetic acid
Purine, uric acid, adenine, guanine.
Structure of nucleic acids

 Pharmacognosy´s topics - Medicinal plants
Pharmacognosy´s topics - Medicinal plants


Write a comment