Theme XVIII: Heterocycles
Hexagonal heterocyclic compounds: The Pyran and Pyrimidine
Characteristics of hexagonal heterocyclic compounds. The Pyran and Pyrimidine. Pyranose structures. Glucose.
Hexagonal heterocyclic compounds
The hexagonal heterocyclic compounds can be presented with 1 or 2 heteroatoms among which are the pyrimidine and pyran.

Pyran

Pyrimidine
Study of Pyran
This compound is a cyclic unsubstituted
hexagonal structure, without charge and with two double bonds, the heteroatom is oxygen and in its position representation should be indicated the two hydrogen atoms to
differentiate the two isomers.

α - Pyran

γ - Pyran
These structures do not present aromatic characteristics and in the system are just 4 π electrons. The pyran derivatives are possible to obtain only through
organic synthesis however appear naturally in numerous compounds.
In the Pyran reduction reaction is first obtained the dihydropyran and then the tetrahydropyran.
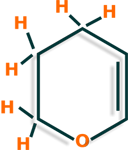
Dihydropyran

Tetrahydropyran
Various organic structures have in their carbon skeleton tetrahydropyran hexagonal rings such carbohydrates, which are glucose polymers. These structures are
called pyranose. For example the structure of glucose.

D glucose
see also
Heterocyclic compounds
Pentagonal heterocyclic compounds with one heteroatom
Pyrrole
Porphyrin. Porphyrin ring
Chlorophyll
Pyrimidine ring
Indole, tryptophan, indole-3-acetic acid
Purine, uric acid, adenine, guanine.
Structure of nucleic acids

 Pharmacognosy´s topics - Medicinal plants
Pharmacognosy´s topics - Medicinal plants


Write a comment